Is Baby Oil a Good Lubricant for Food Machines

Silicone caulk tin exist used as a basic sealant against water and air penetration.
A silicone or polysiloxane is a polymer fabricated up of siloxane (−R2Si−O−SiR2−, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking utensils, thermal insulation, and electrical insulation. Some common forms include silicone oil, silicone grease, silicone rubber, silicone resin, and silicone caulk.[1] [ii]
Chemistry [edit]
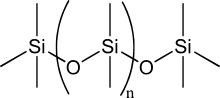
Chemical construction of the silicone polydimethylsiloxane (PDMS)
More than precisely called polymerized siloxanes or polysiloxanes, silicones consist of an inorganic silicon–oxygen backbone chain (⋯−Si−O−Si−O−Si−O−⋯) with two organic groups attached to each silicon centre. Normally, the organic groups are methyl. The materials can exist circadian or polymeric. Past varying the −Si−O− concatenation lengths, side groups, and crosslinking, silicones can be synthesized with a wide variety of properties and compositions. They tin can vary in consistency from liquid to gel to rubber to difficult plastic. The most mutual siloxane is linear polydimethylsiloxane (PDMS), a silicone oil.[ commendation needed ] The second-largest grouping of silicone materials is based on silicone resins, which are formed by branched and muzzle-similar oligosiloxanes.[ citation needed ]
Terminology and history [edit]
F. S. Kipping coined the word silicone in 1901 to describe the formula of polydiphenylsiloxane, Ph2SiO (Ph denoting phenyl, C6H5), by analogy with the formula of the ketone benzophenone, PhtwoCO (his term was originally silicoketone). Kipping was well enlightened that polydiphenylsiloxane is polymeric whereas benzophenone is monomeric and noted the contrasting backdrop of Ph2SiO and PhtwoCO.[3] [four] The discovery of the structural differences between Kipping'south molecules and the ketones means that silicone is no longer the right term (though information technology remains in mutual usage) and that the term siloxane is preferred according to the nomenclature of modern chemistry.[5]
Silicone is often confused with silicon, simply they are distinct substances. Silicon is a element, a hard dark-greyness semiconducting metalloid, which in its crystalline form is used to brand integrated circuits ("electronic chips") and solar cells. Silicones are compounds that contain silicon, carbon, hydrogen, oxygen, and perhaps other kinds of atoms as well, and have many very different physical and chemical properties.
Compounds containing silicon–oxygen double bonds, now chosen silanones, just which could deserve the name "silicone", accept long been identified equally intermediates in gas-phase processes such every bit chemical vapor deposition in microminiaturization product, and in the formation of ceramics past combustion.[6] However, they accept a strong tendency to polymerize into siloxanes. The beginning stable silanone was obtained in 2014 by A. Filippou and others.[vii]
Synthesis [edit]
Nearly common are materials based on polydimethylsiloxane, which is derived by hydrolysis of dimethyldichlorosilane. This dichloride reacts with water equally follows:
- n Si(CHthree)iiClii + north HiiO → [Si(CH3)twoO] n + 2due north HCl
The polymerization typically produces linear chains capped with Si−Cl or Si−OH (silanol) groups. Under dissimilar weather, the polymer is a circadian, not a chain.[one]
For consumer applications such every bit caulks silyl acetates are used instead of silyl chlorides. The hydrolysis of the acetates produces the less dangerous acerb acrid (the acid found in vinegar) as the reaction production of a much slower curing process. This chemical science is used in many consumer applications, such as silicone caulk and adhesives.
- n Si(CH3)2(CHthreeCOO)2 + due north H2O → [Si(CH3)2O] n + twonorthward CH3COOH
Branches or crosslinks in the polymer concatenation can exist introduced by using organosilicone precursors with fewer alkyl groups, such as methyl trichlorosilane and methyltrimethoxysilane. Ideally, each molecule of such a chemical compound becomes a branch betoken. This process tin exist used to produce hard silicone resins. Similarly, precursors with 3 methyl groups can be used to limit molecular weight, since each such molecule has only 1 reactive site and so forms the end of a siloxane chain.
Combustion [edit]
When silicone is burned in air or oxygen, information technology forms solid silica (silicon dioxide, SiO2) as a white powder, char, and various gases. The readily dispersed pulverisation is sometimes called silica fume. The pyrolysis of sure polysiloxanes under an inert atmosphere is a valuable pathway towards the product of amorphous silicon oxycarbide ceramics, as well known as polymer derived ceramics. Polysiloxanes terminated with functional ligands such as vinyl, mercapto or acrylate groups have been cross linked to yield preceramic polymers, which can be photopolymerised for the additive manufacturing of polymer derived ceramics by stereolithography techniques.[eight]
Backdrop [edit]
![]()
This silicone safety folding chessboard resists creasing and wrinkling.
Silicones exhibit many useful characteristics, including:[i]
- Depression thermal conductivity
- Low chemical reactivity
- Low toxicity
- Thermal stability (constancy of properties over a wide temperature range of −100 to 250 °C)
- The ability to repel water and form watertight seals.
- Does not stick to many substrates, simply adheres very well to others, e.g. drinking glass
- Does not support microbiological growth
- Resistance to oxygen, ozone, and ultraviolet (UV) light. This property has led to the widespread employ of silicones in the construction industry (e.one thousand. coatings, burn protection, glazing seals) and the automotive manufacture (external gaskets, external trim).
- Electric insulation properties. Considering silicone can exist formulated to be electrically insulative or conductive, it is suitable for a broad range of electrical applications.
- High gas permeability: at room temperature (25 °C), the permeability of silicone rubber for such gases equally oxygen is approximately 400 times[9] that of butyl rubber, making silicone useful for medical applications in which increased aeration is desired. Conversely, silicone rubbers cannot be used where gas-tight seals are necessary such every bit seals for high-pressure gasses or high vacuum.
Silicone can be developed into rubber sheeting, where it has other backdrop, such every bit being FDA compliant. This extends the uses of silicone sheeting to industries that demand hygiene, for instance, food and drinkable, and pharmaceuticals.
Applications [edit]
Silicones are used in many products. Ullmann's Encyclopedia of Industrial Chemistry lists the following major categories of awarding: Electrical (due east.g. insulation), electronics (e.g., coatings), household (due east.g., sealants and cooking utensils), automobile (east.g. gaskets), airplane (e.thou., seals), office machines (due east.grand. keyboard pads), medicine and dentistry (e.g. molar impression molds), textiles and paper (due east.grand. coatings). For these applications, an estimated 400,000 tonnes of silicones were produced in 1991.[ description needed ] Specific examples, both large and small are presented below.[ane]
Automotive [edit]

Silicone caulks and rubber components are increasingly used in automotive applications.
In the automotive field, silicone grease is typically used as a lubricant for brake components since information technology is stable at loftier temperatures, is non water-soluble, and is far less likely than other lubricants to foul. DOT 5 brake fluids are based on liquid silicones.
Automotive spark plug wires are insulated by multiple layers of silicone to forbid sparks from jumping to next wires, causing misfires. Silicone tubing is sometimes used in automotive intake systems (especially for engines with forced induction).
Sail silicone is used to manufacture gaskets used in automotive engines, transmissions, and other applications.
Automotive body manufacturing plants and pigment shops avoid silicones, equally trace contagion may cause "fish eyes", which are small, circular craters which mar a shine finish.[ citation needed ]
Additionally, silicone compounds such as silicone rubber are used as coatings and sealants for airbags; the high strength of silicone rubber makes information technology an optimal adhesive and sealant for high touch on airbags.[ citation needed ] Silicones in combination with thermoplastics provide improvements in scratch and mar resistance and lowered coefficient of friction.[ commendation needed ]
Aerospace [edit]

Silicone is often used to seal maintenance access openings in aerospace equipment.
Silicone is a widely used textile in the aerospace manufacture due to its sealing properties, stability beyond an extreme temperature range, durability, sound dampening and anti-vibration qualities, and naturally flame retardant properties. Maintaining farthermost functionality is paramount for passenger safety in the aerospace industry, so each component on an aircraft requires loftier-performance materials.
Specially developed aerospace grades of silicone are stable from −lxx to 220 °C,[ten] these grades tin be used in the structure of gaskets for windows and cabin doors. During performance, aircraft become through big temperature fluctuations in a relatively short menses of time; from freezing temperatures when flying at full distance to the ambience temperatures when on the ground in hot countries. Silicone rubber tin be molded with tight tolerances ensuring gaskets form airtight seals both on the basis and in the air, where atmospheric pressure decreases.
Silicone safety'south resistance to heat corrosion enables it to exist used for gaskets in aircraft engines where it will outlast other types of rubber, both improving aircraft prophylactic and reducing maintenance costs. The silicone acts to seal instrument panels and other electrical systems in the cockpit, protecting printed circuit boards from the risks of farthermost altitude such as wet and extremely low temperature. Silicone can be used equally a sheath to protect wires and electrical components from whatsoever dust or ice that may creep into a plane's inner workings.
As the nature of air travel results in much racket and vibration, powerful engines, landings, and high speeds all need to be considered to ensure passenger comfort and safe operation of the shipping. As silicone rubber has exceptional dissonance reduction and anti-vibration properties, it can be formed into small components and fitted into small gaps ensuring all equipment tin exist protected from unwanted vibration such every bit overhead lockers, vent ducts, hatches, entertainment system seals, and LED lighting systems.
Building construction [edit]
The strength and reliability of silicone rubber are widely best-selling in the construction industry. One-office silicone sealants and caulks are in common apply to seal gaps, joints and crevices in buildings. One-part silicones cure by absorbing atmospheric moisture, which simplifies installation. In plumbing, silicone grease is typically applied to O-rings in contumely taps and valves, preventing lime from sticking to the metal.
Structural silicone has besides been used in drape wall building façades since 1974 when the Art Establish of Chicago became the showtime building to receive outside glass fixed just with the material.[ citation needed ] Silicone membranes have been used to encompass and restore industrial roofs, thank you to its farthermost UV resistance, and power to go on their waterproof performance for decades.[ commendation needed ]
Coatings [edit]
Silicone films tin can be applied to such silica-based substrates every bit drinking glass to form a covalently bonded hydrophobic coating. Such coatings were developed for employ on aircraft windshields to repel water and to preserve visibility, without requiring mechanical windshield wipers which are impractical at supersonic speeds. Like treatments were eventually adapted to the automotive market in products marketed by Rain-X and others.
Many fabrics can be coated or impregnated with silicone to grade a strong, waterproof composite such as silnylon.
A silicone polymer tin can be suspended in water by using stabilizing surfactants. This allows water-based formulations to be used to deliver many ingredients that would otherwise require a stronger solvent, or be as well viscous to utilise finer. For example a waterborne formulation using a silane'due south reactivity and penetration power into a mineral-based surface tin can be combined with h2o-beading backdrop from a siloxane to produce a more-useful surface protection product.
Cookware [edit]
As a low-taint, non-toxic material, silicone can be used where contact with food is required. Silicone is becoming an of import production in the cookware industry, particularly bakeware and kitchen utensils. Silicone is used as an insulator in heat-resistant potholders and similar items; however, it is more than conductive of heat than like less dumbo fiber-based products. Silicone oven mitts are able to withstand temperatures up to 260 °C (500 °F), making information technology possible to reach into boiling water.
Other products include molds for chocolate, ice, cookies, muffins, and various other foods; non-stick bakeware and reusable mats used on baking sheets; steamers, egg boilers or poachers; cookware lids, pot holders, trivets, and kitchen mats.
-

Soup ladle and pasta ladle made of silicone
-

A silicone food steamer to be placed within a pot of boiling water
-

Flexible water ice cube trays made of silicone allow piece of cake extraction of water ice.
-

Silicone brush used for basting and applying flavoring liquids
Defoaming [edit]
Silicones are used as agile compounds in defoamers due to their depression water solubility and good spreading properties.
Dry cleaning [edit]
Liquid silicone can exist used every bit a dry cleaning solvent, providing an culling to the traditional chlorine-containing perchloroethylene (perc) solvent. The use of silicones in dry out cleaning reduces the environmental effect of a typically loftier-polluting manufacture.[ citation needed ]
Electronics [edit]
![]()
Electronic components are sometimes encased in silicone to increase stability confronting mechanical and electrical shock, radiations and vibration, a process called "potting". Silicones are used where durability and high performance are demanded of components under extreme environmental atmospheric condition, such every bit in space (satellite technology). They are selected over polyurethane or epoxy encapsulation when a broad operating temperature range is required (−65 to 315 °C). Silicones also have the advantage of trivial exothermic heat ascent during cure, low toxicity, proficient electrical properties, and high purity.
Silicones are frequently a component of thermal pastes used to improve heat transfer from power-dissipating electronic components to heat sinks.
The apply of silicones in electronics is non without problems, however. Silicones are relatively expensive and can be attacked by certain solvents. Silicone easily migrates every bit either a liquid or vapor onto other components. Silicone contamination of electrical switch contacts can lead to failures past causing an increase in contact resistance, often belatedly in the life of the contact, well after whatever testing is completed.[eleven] [12] Utilise of silicone-based spray products in electronic devices during maintenance or repairs can cause later on failures.
Firestops [edit]
![]()
Ruby-red-colored silicone firestopping
Silicone foam has been used in North American buildings in an attempt to firestop openings within the fire-resistance-rated wall and floor assemblies to preclude the spread of flames and fume from one room to some other. When properly installed, silicone-cream firestops tin can be fabricated for building code compliance. Advantages include flexibility and loftier dielectric strength. Disadvantages include combustibility (difficult to extinguish) and significant smoke development.
Silicone-foam firestops take been the subject of controversy and printing attending due to smoke evolution from pyrolysis of flammable components inside the foam, hydrogen gas escape, shrinkage, and peachy. These issues take led to reportable events among licensees (operators of nuclear power plants) of the Nuclear Regulatory Commission (NRC).[ citation needed ]
Silicone firestops are also used in aircraft.
Jewelry [edit]
Silicone is a popular alternative to traditional metals (such as silver and aureate) with jewelry, specifically rings. Silicone rings are commonly worn in professions where metal rings tin can lead to injuries, such equally electric conduction and ring avulsions.[xiii] [14] During the mid-2010's, some professional person athletes began wearing silicone rings as an culling during games.[15]
Lubricants [edit]

Silicone grease is often used with laboratory glassware to preclude seizing.
Silicone greases are used for many purposes, such as bicycle bondage, airsoft gun parts, and a wide range of other mechanisms. Typically, a dry-set lubricant is delivered with a solvent carrier to penetrate the mechanism. The solvent then evaporates, leaving a articulate motion picture that lubricates merely does not attract dirt and dust every bit much equally an oil-based or other traditional "wet" lubricant.
Silicone personal lubricants are also bachelor for use in medical procedures or sexual activity.
Medicine and cosmetic surgery [edit]
Silicone is used in microfluidics, seals, gaskets, shrouds, and other applications requiring high biocompatibility. Additionally, the gel form is used in bandages and dressings, breast implants, testicle implants, pectoral implants, contact lenses, and a multifariousness of other medical uses.
Scar treatment sheets are often made of medical grade silicone due to its durability and biocompatibility. Polydimethylsiloxane (PDMS) is often used for this purpose, since its specific crosslinking results in a flexible and soft silicone with high durability and tack. It has likewise been used as the hydrophobic cake of amphiphilic synthetic block copolymers used to form the vesicle membrane of polymersomes.
Illicit cosmetic silicone injections may induce chronic and definitive silicone claret diffusion with dermatologic complications.[16]
Ophthamology uses many products such as silicone oil used to replace the vitreous humor post-obit vitrectomy, silicone intraocular lenses post-obit cataract extraction, silicone tubes to keep a nasolacrimal passage open following dacryocystorhinostomy, canalicular stents for canalicular stenosis, punctual plugs for punctual occlusion in dry out eyes, silicone rubber and bands as an external tamponade in tractional retinal disengagement, and anteriorly-located suspension in rhegmatogenous retinal disengagement.
Moldmaking [edit]
![]()
Silicone mold used to reproduce an architectural detail
Two-part silicone systems are used equally rubber molds to cast resins, foams, rubber, and low-temperature alloys. A silicone mold generally requires piddling or no mold-release or surface training, every bit virtually materials do not adhere to silicone. For experimental uses, ordinary ane-part silicone can exist used to brand molds or to mold into shapes. If needed, common vegetable cooking oils or petroleum jelly tin exist used on mating surfaces as a mold-release agent.[17]
Silicone cooking molds used as bakeware do not require coating with cooking oil; in improver, the flexibility of the rubber allows the baked food to be easily removed from the mold afterward cooking.
Personal care [edit]

Silicone prophylactic earplugs for hearing protection
Silicones are ingredients widely used in skincare, color cosmetic and pilus care applications. Some silicones, notably the amine functionalized amodimethicones, are excellent hair conditioners, providing improved compatibility, feel, and softness, and lessening frizz. The phenyl dimethicones, in another silicone family, are used in reflection-enhancing and color-correcting hair products, where they increase shine and glossiness (and possibly impart subtle color changes). Phenyltrimethicones, unlike the workout amodimethicones, have refractive indices (typically i.46) close to that of a human hair (i.54). However, if included in the same conception, amodimethicone and phenyltrimethicone interact and dilute each other, making information technology difficult to achieve both high shine and excellent conditioning in the same product.[eighteen]
Silicone rubber is commonly used in babe bottle nipples (teats) for its cleanliness, aesthetic appearance, and low extractable content.
Silicones are used in shaving products and personal lubricants.[19]
Toys and hobbies [edit]
![]()
Babe toys made of nontoxic silicone safety
Light-headed Putty and like materials are equanimous of silicones dimethyl siloxane, polydimethylsiloxane, and decamethyl cyclopentasiloxane, with other ingredients. This substance is noted for its unusual characteristics, e.g., that it bounces, but breaks when given a sharp blow; it volition also menstruum like a liquid and form a puddle given plenty time.
Silicone "rubber bands" are a long-lasting popular replacement refill for real rubber bands in the 2013 fad "rubber band loom" toys at two to four times the cost (in 2014). Silicone bands besides come up in bracelet sizes that can be custom embossed with a proper name or message. Big silicone bands are also sold as utility necktie-downs.
Formerol is a silicone safe (marketed as Sugru) used every bit an arts-and-crafts cloth, as its plasticity allows it to be molded by mitt like modeling clay. It hardens at room temperature and it is agglutinative to various substances including drinking glass and aluminum.[20]
Oogoo is a inexpensive silicone clay, which can be used as a substitute for Sugru.[21]
In making aquariums, manufacturers at present commonly apply 100% silicone sealant to join glass plates. Glass joints made with silicone sealant tin can withstand great pressure level, making obsolete the original aquarium structure method of angle-fe and putty. This same silicone is used to brand hinges in aquarium lids or for small repairs. However, non all commercial silicones are safe for aquarium manufacture, nor is silicone used for the manufacture of acrylic aquariums equally silicones practise not have long-term adhesion to plastics.[22]
Production and marketing [edit]
The global demand for silicones approached The states$12.5 billion in 2008, approximately 4% up from the previous year. Information technology continues similar growth in the following years to reach $thirteen.5 billion by 2010. The annual growth is expected to be additional by broader applications, introduction of novel products and increasing sensation of using more environmentally friendly materials.[23]
The leading global manufacturers of silicone base of operations materials vest to three regional organizations: the European Silicone Center (CES) in Brussels, Belgium; the Environment Health and Rubber Quango (SEHSC) in Herndon, Virginia, The states; and the Silicone Industry Association of Nippon (SIAJ) in Tokyo, Nippon. Dow Corning Silicones, Evonik Industries, Momentive Performance Materials, Milliken and Company (SiVance Specialty Silicones), Shin-Etsu Silicones, Wacker Chemie, Bluestar Silicones, JNC Corporation, Wacker Asahikasei Silicone, and Dow Corning Toray correspond the commonage membership of these organizations. A quaternary organization, the Global Silicone Council (GSC) acts as an umbrella structure over the regional organizations. All four are non-turn a profit, having no commercial function; their primary missions are to promote the safety of silicones from a health, safety, and environmental perspective. As the European chemical manufacture is preparing to implement the Registration, Evaluation, and Authorisation of Chemicals (Accomplish) legislation, CES is leading the germination of a consortium[24] of silicones, silanes, and siloxanes producers and importers to facilitate data and toll-sharing.
Safety and environmental considerations [edit]
Silicone compounds are pervasive in the environment. Item silicone compounds, cyclic siloxanes Dfour and D5, are air and water pollutants and have negative wellness effects on examination animals.[25] They are used in various personal care products. The European Chemicals Agency found that "D4 is a persistent, bioaccumulative and toxic (PBT) substance and D5 is a very persistent, very bioaccumulative (vPvB) substance".[26] [27] Other silicones biodegrade readily, a procedure that is accelerated past a multifariousness of catalysts, including clays.[1] Cyclic silicones have been shown to involve the occurrence of silanols during biodegradation in mammals.[28] The resulting silanediols and silanetriols are capable of inhibiting hydrolytic enzymes such as thermolysin, acetycholinesterase, however, the doses required for inhibition are by orders of magnitude higher than the ones resulting from the accumulated exposure to consumer products containing cyclomethicone.[29] [thirty]
At around 200 °C (392 °F) in an oxygen-containing atmosphere, PDMS releases traces of formaldehyde (but lesser amounts than other mutual materials such as polyethylene.[31] [32]) At this temperature, silicones were found to have lower formaldehyde generation than mineral oil and plastics (less than three to 48 µg CH2O/(g·hr) for a loftier consistency silicone condom, versus around 400 µg CHiiO/(grand·hour) for plastics and mineral oil). By 250 °C (482 °F), copious amounts of formaldehyde accept been found to exist produced by all silicones (1,200 to iv,600 µg CHtwoO/(m·hr)).[32]
See also [edit]
- Injection molding of liquid silicone rubber
References [edit]
- ^ a b c d e Moretto, Hans-Heinrich; Schulze, Manfred; Wagner, Gebhard (2005). "Silicones". Ullmann'southward Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:x.1002/14356007.a24_057.
- ^ Fink, Johannes Karl (5 July 2019). Liquid Silicone Rubber: Chemistry, Materials, and Processing. ISBN9781119631378.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 362. ISBN978-0-08-037941-viii.
- ^ Frederic Kipping, L. L. Lloyd (1901). "XLVII. Organic derivatives of silicon. Triphenylsilicol and alkyloxysilicon chlorides". J. Chem. Soc., Trans. 79: 449–459. doi:10.1039/CT9017900449.
- ^ James E. Marker; Harry R. Allcock; Robert West (24 March 2005). Inorganic Polymers. Oxford University. p. 155. ISBN978-0-19-535131-6. Archived from the original on 18 Dec 2017.
- ^ V. Due north. Khabashesku; Z. A. Kerzina; K. Due north. Kudin; O. M. Nefedov (1998). "Matrix isolation infrared and density functional theoretical studies of organic silanones, (CH3O)2Si=O and (CviH5)2Si=O". J. Organomet. Chem. 566 (1–2): 45–59. doi:10.1016/S0022-328X(98)00726-8.
- ^ Alexander C. Filippou, Bernhard Baars, Yury N. Lebedev, and Gregor Schnakenburg (2014): "Silicon–Oxygen Double Bonds: A Stable Silanone with a Trigonal‐Planar Coordinated Silicon Middle". Angewandte Chemie International Edition, book 53, outcome two, pages 565–570. doi:x.1002/anie.201308433.
- ^ Additive manufacturing of ceramics from preceramic polymers: A versatile stereolithographic approach assisted by thiol-ene click chemistry. Additive Manufacturing, (2019) volume 27, pp. eighty–90.
- ^ "Treeing Characteristics in HTV Silicone Rubber", Electrical Insulation Breakdown and Its Theory, Process, and Prevention, Advances in Calculator and Electrical Engineering, IGI Global, pp. 73–104, 2020, doi:x.4018/978-1-5225-8885-6.ch003, ISBN978-1-5225-8885-6 , retrieved 2021-03-sixteen
- ^ "Aerospace | Viking Extrusions". www.vikingextrusions.co.u.k. . Retrieved 2019-04-11 .
- ^ Paul Thou. Slade (1999). "16.iv.one". Electrical Contacts: Principles and Applications. CRC Press. p. 823. ISBN978-0-8247-1934-0. Archived from the original on 2017-12-xviii.
- ^ W. Witter & R. Leiper (1979). "A Comparison for the Effects of Various Forms of Silicon Contamination on Contact Performance". IEEE Transactions on Components, Hybrids, and Manufacturing Applied science. 2: 56–61. doi:10.1109/TCHMT.1979.1135411.
- ^ Ashley, Sarah (August 1, 2018). "Is the Silicone Ring Trend Here to Stay?".
- ^ Chen, Connie. "A ton of couples are skipping the fancy wedding ceremony rings and opting for these $twenty rubber bands instead — here'south why". Insider.
- ^ "The (hymeneals) ring's the thing: Silicone bands a growing trend in NFL". ESPN.com. September 29, 2016.
- ^ Bertin, Chloé; Abbas, Rachid; Andrieu, Valérie; Michard, Florence; Rioux, Christophe; Descamps, Vincent; Yazdanpanah, Yazdan; Bouscarat, Fabrice (January 1, 2019). "Illicit massive silicone injections ever induce chronic and definitive silicone claret diffusion with dermatologic complications". Medicine. 98 (4): e14143. doi:10.1097/Dr..0000000000014143 – via journals.lww.com.
- ^ Robyn Lish. What are the benefits of Silicone Caulk Moulds. Myheap.com. Retrieved on 2021-08-08.
- ^ Thomas Clausen et al. "Pilus Preparations" in Ullmann'south Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. doi:10.1002/14356007.a12_571.pub2
- ^ Q. Ashton Acton: Silicones—Advances in Research and Application: 2013 Edition, ScholarlyEditions, 2013, ISBN 9781481692397, p. 226 Archived 2017-12-xviii at the Wayback Motorcar.
- ^ " "Formerol/Sugru technical information sheet".
- ^ "How to Make Your Own Sugru Substitute". Instructables.
- ^ "Aquarium Silicone Applications". Aquarium-pond-answers.com. March 2007. Archived from the original on 2012-03-xv. Retrieved 2012-02-28 .
- ^ "Market place Written report: World Silicone Marketplace". Acmite Market place Intelligence. Archived from the original on 2010-09-14.
- ^ "REACH consortium". Reach.silicones.eu. Archived from the original on 2012-03-15. Retrieved 2012-02-28 .
- ^ Bienkowski, Brian (30 Apr 2013). "Chemicals from Personal Care Products Pervasive in Chicago Air". Scientific American. Archived from the original on 20 June 2015. Retrieved 8 Apr 2015.
- ^ European Chemicals Agency. "Committee for Risk Assessment concludes on restricting D4 and D5". European Chemicals Agency. Retrieved 28 Baronial 2018.
- ^ "ECHA classifies cyclic siloxanes as SVHCs". Food Packaging Forum Foundation. Retrieved 28 August 2018.
- ^ Southward. Varaprath, K. L. Salyers, K. P. Plotzke and S. Nanavati: "Identification of Metabolites of Octamethylcyclotetrasiloxane (D4) in Rat Urine", Drug Metab Dispos 1999, 27, 1267-1273.
- ^ S. M. Sieburth, T. Nittoli, A. Thou. Mutahi and L. Guo: Silanediols: a new class of potent protease inhibitors, Angew. Chem. Int. Ed. 1998, volume 37, 812-814.
- ^ Yard. Blunder, N. Hurkes, M. Listing, South. Spirk and R. Pietschnig: Silanetriols as in vitro AChE Inhibitors, Bioorg. Med. Chem. Lett. 2011, volume 21, 363-365.
- ^ Hard, Dave. "Dielectric Fluids for Transformer Cooling — History and Types". General Electrical. Archived from the original on 2016-07-19.
- ^ a b David C. Timpe Jr. Formaldehyde Generation from Silicone Rubber Archived 2015-04-27 at the Wayback Machine Arlon
External links [edit]
| | Look up silicone in Wiktionary, the gratis dictionary. |
| | Wikimedia Eatables has media related to Silicones. |
- Science of Silicone Polymers (Silicone Science On-line, Eye Européen des Silicones: CES)
- Is Silicone Eco-friendly? (The Uptide)
Source: https://en.wikipedia.org/wiki/Silicone
0 Response to "Is Baby Oil a Good Lubricant for Food Machines"
Enregistrer un commentaire